 Your new post is loading...
 Your new post is loading...
Riesgo de hospitalización y de infecciones en pacientes con artritis reumatoide tratados con biológicos y denosumab http://t.co/cLLGPSJZL6 Abstract Objective Denosumab is a biologic agent used to treat osteoporosis. Its safety profile given concurrently with biologic drugs for rheumatoid arthritis (RA) has not been well studied. We evaluated hospitalized infections among patients treated with biologic agents for RA who initiated denosumab or zoledronic acid (ZA), a parenteral bisphosphonate without known associations with infection. We hypothesized that the rate of hospitalized infection with denosumab would be noninferior to ZA. Methods We identified RA patients enrolled in Medicare in 2006–2012 treated with biologic agents who initiated denosumab or ZA. Cox proportional hazards models compared the risk for hospitalized infection, comparing denosumab users to ZA users and adjusting for potentially confounding factors. A noninferiority margin was specified a priori to demonstrate that denosumab had no greater infection risk than ZA if the upper bound of the 95% confidence interval (95% CI) of the hazard ratio (HR) was <1.5. Results Eligible RA patients receiving biologic agents initiated denosumab (n = 1,354) or ZA (n = 4,460). Characteristics of the denosumab users were as follows: mean ± SD age 73.0 ± 8.9, 98.2% women, with a majority receiving infliximab (35.7%) or abatacept (18.6%). Denosumab users had a higher prevalence of prior infections (11.5% hospitalized and 48.3% outpatient) and infection-related risk factors. The crude rate of hospitalized infections for denosumab (14.9/100 person-years [95% CI 12.2–18.1]) was comparable to that for ZA (13.9/100 person-years [95% CI 12.5–15.4]). After adjustment, the HR of hospitalized infection for denosumab users was noninferior to that for ZA users (HR 0.89 [95% CI 0.69–1.15]). Conclusion The rate of hospitalized infection among RA patients receiving denosumab concurrently with biologic agents for RA was not increased compared to those receiving zoledronate.
Via Krishan Maggon
C-reactive protein (CRP) is one of the biomarkers for the diagnosis and assessment of disease activity in rheumatoid arthritis (RA). CRP is not only the by-product of inflammatory response, but also plays pro-inflammatory and pro-thrombotic roles.
Via Krishan Maggon
An evaluation of risk factors for major adverse #cardiovascular events during #tocilizumab therapy http://t.co/EymLTiXiv1 #rheum Abstract Objective. To explore associations of baseline and on-treatment lipid levels, inflammation, and rheumatoid arthritis (RA) disease activity with risk for major adverse cardiovascular events (MACE) in tocilizumab-treated RA patients. Methods. In retrospective post hoc analyses, data were pooled from 3986 adults with moderate to severe RA administered ≥1 dose of tocilizumab 4 or 8 mg/kg intravenously every 4 weeks in randomized controlled trials and extension studies. Cox proportional hazards modeling was used to evaluate associations among baseline characteristics and posttreatment initiation variables (week 24) and change from baseline to week 24 disease activity and laboratory values, with risk for future MACE during extended follow-up. Results. There were 50 independently adjudicated cases of MACE during 14,683 patient-years (PY) of follow-up (0.34 MACE/100 PY). At baseline, age, history of cardiac disorders, disease activity score using 28 joints (DAS28), and total cholesterol/high-density lipoprotein ratio were independently (P<0.05 for all) associated with MACE in multivariable models. On treatment, higher DAS28 scores and swollen and tender joint counts at week 24 were associated with future MACE. In separate models, greater reductions in DAS28 score and joint counts from baseline to week 24 were inversely associated with future MACE; changes in lipid parameters were not statistically significantly associated with risk for MACE. Conclusion. As in the general population, an association was observed between baseline total cholesterol/high-density lipoprotein ratio and increased risk for MACE. Risk for on-treatment MACE, however, was found to be associated with control of disease activity but not lipid changes. Larger studies are needed to confirm these findings. © 2014 American College of Rheumatology.
Via Krishan Maggon
Abstract Objectives The safety and efficacy of sirukumab, an anti-interleukin-6 (IL-6) monoclonal antibody, were evaluated in a 2-part, placebo-controlled phase II study of patients with active rheumatoid arthritis (RA) despite methotrexate therapy. Methods In Part A (proof-of-concept), 36 patients were randomised to placebo or sirukumab 100 mg every 2 weeks (q2w) through week 10, with crossover treatment during weeks 12–22. In Part B (dose finding), 151 patients were randomised to sirukumab (100 mg q2w, 100 mg q4w, 50 mg q4w, or 25 mg q4w) through week 24, or placebo through week 10 with crossover to sirukumab 100 mg q2w (weeks 12–24). The proportion of patients with an American College of Rheumatology 50 (ACR50) response and the change from baseline in the 28-joint count disease activity score using C-reactive protein (DAS28-CRP) were determined. Safety was evaluated through week 38 in both parts. Results The primary endpoint (ACR50 at week 12 in Part B) was achieved only with sirukumab 100 mg q2w versus placebo (26.7% vs 3.3%; p=0.026). Greater improvements in mean DAS28-CRP at week 12 were observed with sirukumab 100 mg q2w versus placebo in Parts A (2.1 vs 0.6, p<0.001) and B (2.2 vs 1.1; p<0.001). The incidence of adverse events (AEs) was similar for sirukumab-treated and placebo-treated patients through week 12 in Part A (70.6% and 63.2%, respectively) and B (67.8% and 66.7%, respectively). Infections were the most common type of AE; one death occurred (Part B, sirukumab 100 mg q2w, brain aneurysm). Conclusions Sirukumab-treated patients experienced improvements in the signs/symptoms of RA. Safety results through 38 weeks were consistent with other IL-6 inhibitors. Trial registration number NCT00718718.
Via Krishan Maggon
The safety and efficacy profiles in clinical trials of olokizumab, sarilumab and sirukumab are similar and are consistent with those observed in RA patients treated with tocilizumab. Furthermore, the clinical efficacy of these IL-6 inhibitors is similar to that of TNF inhibitors in patients with MTX-IR and TNF-IR. Screening of biomarkers or genetics in each RA patient, for instance, baseline serum levels of TNF and/or soluble IL-6R, may help to predict the efficacy of each drug and to select patients for cytokine-oriented targeted therapies.33 However, better strategies are warranted for selecting and identifying appropriate patients earlier once bDMARDs targeting IL-6 are launched in the near future. We also need to determine whether there are important differences between the many IL-6 inhibitors and which are suitable for particular patients, otherwise companies may waste time and money in development.
Via Krishan Maggon
|
Tocilizumab was safe and effective for RA patients in comparison to tocilizumab plus DMARD therapy.
Via Krishan Maggon
Hospitalized Infection rates among BiologicTreated RA Patients Concurrently Treated with Denosumab A & R http://t.co/D6FE0QbuaL Methods We identified RA patients enrolled in Medicare in 2006-2012 treated with biologics who initiated Dmab or ZA. Cox proportional hazards models compared the risk for hospitalized infection, comparing denosumab to ZA users and adjusting for potentially confounding factors . A non-inferiority margin was specified a-priori to demonstrate that denosumab had no greater infection risk than ZA if the upper bound of the 95% CI of the hazard ratio (HR) was less than 1.5. Results Eligible RA patients receiving biologics initiated denosumab (n=1,354) or ZA (n=4,460). Characteristics of the denosumab users: mean (SD) age 73.0(8.9), 98.2% women, with a majority receiving infliximab (35.7%) or abatacept (18.6%). Denosumab users had a higher prevalence of prior infections (11.5% hospitalized, 48.3% outpatient) and infection-related risk factors. The crude rate of hospitalized infection for Dmab (14.9/100py; 95% CI 12.2-18.1) was comparable to ZA (13.9/100py; 95% CI 12.5-15.4). After adjustment, the HR of hospitalized infection for denosumab users was non-inferior to ZA (HR=0.89, 95% CI 0.69 - 1.15) Conclusion The rate of hospitalized infection among RA patients receiving denosumab concurrently with biologics for RA was not increased compared to zoledronate.
Via Krishan Maggon
Abstract Objectives To evaluate safety and efficacy of weekly (qw) and every other week (q2w) dosing of sarilumab, a fully human anti-interleukin 6 receptor α (anti-IL-6Rα) monoclonal antibody, for moderate-to-severe rheumatoid arthritis (RA). Methods In this dose-ranging study, patients (n=306) with active RA, despite methotrexate, were randomly assigned to placebo or one of five subcutaneous doses/regimens of sarilumab: 100 mg q2w, 150 mg q2w, 100 mg qw, 200 mg q2w, 150 mg qw for 12 weeks, plus methotrexate. The primary end point was ACR20 at Week 12. Secondary endpoints included ACR50, ACR70, Disease Activity Score in 28 joints (C reactive protein). Safety, pharmacokinetics, pharmacodynamics and efficacy in population subgroups were assessed. Results The proportion of patients achieving an ACR20 response compared with placebo was significantly higher for sarilumab 150 mg qw (72.0% vs 46.2%, multiplicity adjusted p=0.0203). Higher ACR20 responses were also attained with 150 mg q2w (67%; unadjusted (nominal) p=0.0363) and 200 mg q2w (65%; unadjusted p=0.0426) versus placebo. Sarilumab ≥150 mg q2w reduced C reactive protein, which did not return to baseline between dosing intervals. Infections were the most common adverse event; none were serious. Changes in laboratory values (neutropenia, transaminases and lipids) were consistent with reports with other IL-6Rα inhibitors. Conclusions Sarilumab improved signs and symptoms of RA over 12 weeks in patients with moderate-to-severe RA with a safety profile similar to reports with other IL-6 inhibitors. Sarilumab 150 mg and sarilumab 200 mg q2w had the most favourable efficacy, safety and dosing convenience and are being further evaluated in Phase III.
Via Krishan Maggon
Abstract Objectives The aim of this 12-week Phase IIb study was to assess the efficacy and safety of olokizumab (OKZ), a humanised anti-IL6 monoclonal antibody, in patients with rheumatoid arthritis (RA) with moderate-to-severe disease activity who had previously failed tumour necrosis factor (TNF) inhibitor therapy. The dose-exposure-response relationship for OKZ was also investigated. Methods Patients were randomised to one of nine treatment arms receiving placebo (PBO) or OKZ (60, 120 or 240 mg) every 4 weeks (Q4W) or every 2 weeks (Q2W), or 8 mg/kg tocilizumab (TCZ) Q4W. The primary endpoint was change from baseline in DAS28(C-reactive protein, CRP) at Week 12. Secondary efficacy endpoints were American College of Rheumatology 20 (ACR20), ACR50 and ACR70 response rates at Week 12. Exploratory analyses included comparisons of OKZ efficacy with TCZ. Results Across 221 randomised patients, OKZ treatment produced significantly greater reductions in DAS28(CRP) from baseline levels at Week 12, compared to PBO (p<0.001), at all the OKZ doses tested (60 mg OKZ p=0.0001, 120 and 240 mg OKZ p<0.0001). Additionally, ACR20 and ACR50 responses were numerically higher for OKZ than PBO (ACR20: PBO=17.1–29.9%, OKZ=32.5–60.7%; ACR50: PBO=1.3–4.9%, OKZ=11.5–33.2%). OKZ treatment, at several doses, demonstrated similar efficacy to TCZ across multiple endpoints. Most adverse events were mild or moderate and comparable between OKZ and TCZ treatment groups. Pharmacokinetic/pharmacodynamic modelling demonstrated a shallow dose/exposure response relationship in terms of percentage of patients with DAS28(CRP) <2.6. Conclusions OKZ produced significantly greater reductions in DAS28(CRP) from baseline at Week 12 compared with PBO. Reported AEs were consistent with the safety profile expected of this class of drug, with no new safety signals identified.
Via Krishan Maggon
Rheumatoid arthritis is a condition that causes painful inflammation of several joints in the body. The joint capsule becomes swollen, and the disease can also destroy cartilage and bone as it progresses. Rheumatoid arthritis affects 0.5% to 1% of the world’s population. Up to this point, doctors have used various drugs to slow or stop the progression of the disease. But now, ETH Zurich researchers have developed a therapy that takes the treatment of rheumatoid arthritis in mice to a new level: after receiving the medication, researchers consider the animals to be fully cured. The drug is a biotechnologically produced active substance consisting of two fused components. One component is the body’s own immune messenger interleukin 4 (IL-4); previous studies have shown that this messenger protects mice with rheumatoid arthritis against cartilage and bone damage. ETH scientists have coupled an antibody to IL-4 that, based on the key-lock principle, binds to a form of a protein that is found only in inflamed tissue in certain diseases (and in tumour tissue). The researchers tested the new fusion molecule, which they refer to as an ‘armed antibody’, in a CTI project together with the ETH spin-off Philochem. They used a mouse model in which the animals developed swollen, inflamed toes and paws within a few days. Among other things, the researchers studied the fusion molecule in combination with dexamethasone, a cortisone-like anti-inflammatory drug that is already used to treat rheumatoid arthritis in humans. The researchers started treating each mouse as soon as they began showing signs of the disease in the form of swollen extremities. Clinical trials in the next year When used separately, the new fusion molecule and dexamethasone managed only to slow the progression of the disease in the affected animals. In contrast, the typical signs of arthritis, such as swollen toes and paws, disappeared completely within a few days when both medications were administered at the same time. Concentrations of a whole range of immune messengers in blood and inflamed tissue, which are changed in rheumatoid arthritis, returned to their normal levels. “In our mouse model, this combined treatment creates a long-term cure,” says Hemmerle, who, since completing her dissertation, has been working at Philochem, where she continues the project.
Via Krishan Maggon
|



 Your new post is loading...
Your new post is loading...





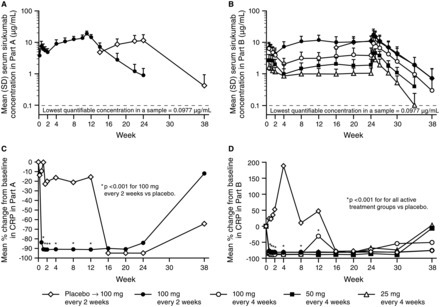


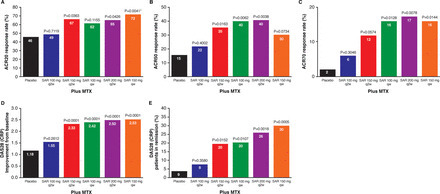
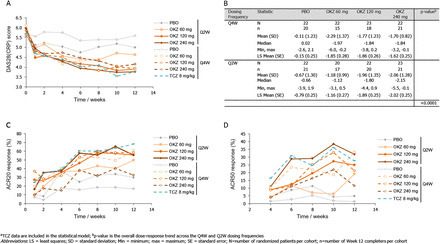




Article first published online: 25 MAY 2015
DOI: 10.1002/art.39075
© 2015, American College of Rheumatology
Issue
Arthritis & RheumatologyVolume 67, Issue 6, pages 1456–1464, June 2015